Diseases of the cardiovascular system rank top in the globe in terms of prevalence and mortality toll. The successful treatment of these disorders necessitates the development of substantially new ways of cardiac implantation as well as novel vascular prosthetic technologies.
According to official figures, over 130 thousand cardiovascular procedures were conducted in Russia alone in 2018. Every year, several arterial reconstructions involving vascular implants are performed in addition to the regular surgical treatments. One of the most typical issues with vascular prostheses is the inflection of the vascular implant at the sites of physiological bends in the joints, which frequently results in a blood flow violation. Surgeons utilise reinforced-wall prostheses to avert such a problem. There is a possibility of profuse bleeding through the walls of the prosthesis following its insertion into the bloodstream if the prosthesis does not conform to the norm (leakage).
As a result, the production of a vascular implant necessitates not only high-quality compliance with medical criteria, but also high-precision porosity testing (surgical porosity). At the time, vascular endoprostheses are being manufactured in Russia, and while various simple implantation solutions are available on the Russian market, a substantial number of patients require more complicated and effective goods to restore the fundamental functioning of the heart and blood arteries.

Sample of prosthetic blood vessel
Such problems need novel methods and a varied set of capabilities, which were made feasible by the ART-UP Design Studio's collaboration with Medtechnoproekt, with assistance from the Skolkovo Foundation and the R&D Services Department.
Medtechnoproject is a Skolkovo resident in the Biomed Cluster, where it develops and implements technologies for the complete cycle manufacture of textile vascular endoprostheses for general and cardiovascular surgery, traumatology, and other applications.
Since 2014, our firm, ART UP Studio (ART-UP Design Studio), has held the official status of the Skolkovo Collective Use Center, delivering industrial design and prototype services to technopark members under the Skolkovo Foundation co-financing initiative.
How did it all begin?
The ART-UP Design Studio was tasked with developing a method for attaching a polymer reinforcing spiral to vascular prosthesis, enclosing them in a device with no analogues in Russia or the globe.
Because it was necessary to design a new sort of equipment to carry out such a work, the project team came up with the notion of employing additive technologies for the task as a result of pre-project study. We opted to use the material application approach, supplemented with the ability to rotate the surface of a given item (in our case, the surface of vascular implants).
The second duty was to inspect the quality of the resultant reinforcing spiral as well as the material of vascular beds created by specialist manufacturers for the fabrication of vascular endoprostheses.
As a result, it became clear that before beginning the reinforcement stage, a preliminary check of the specialised material for porosity was required, and that after applying the spiral, the ready-made reinforced vascular bed required checking for tightness and the ability to withstand a given internal pressure.
Following a conceptual engineering research, it was determined to develop two devices with separate functional purposes that would be linked into a single hardware complex.
The complex's composition:
- A device for applying and fastening a biopolymer coating with an anti-thrombogenic coating to the surface of textile-based vascular prosthesis, as well as for reinforcing the walls of tubular vascular prostheses. The device buttresses the vascular prosthesis, providing flexibility, elasticity, and fracture resistance.
- A method for assessing the degree of porosity of textile-based vascular prostheses impregnated with a biopolymer composition estimates the surgical porosity of a vascular prosthesis at a medium pressure of 120-160 mmHg.
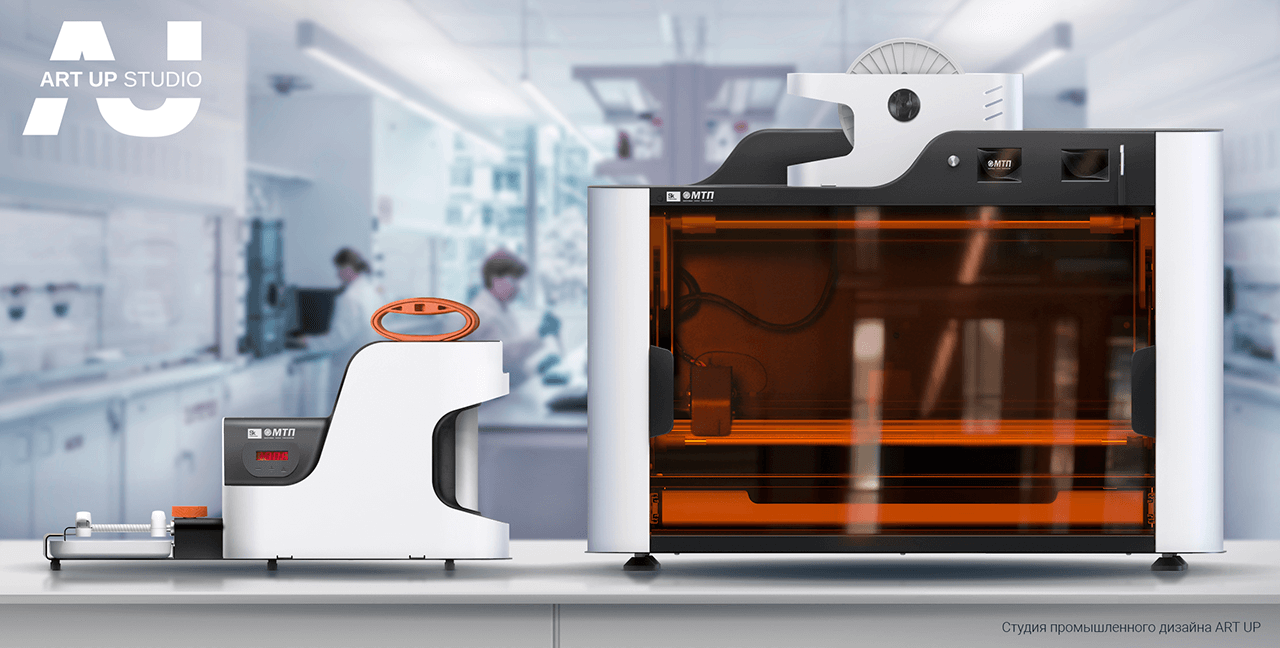
The first product is a device for applying and securing a polymer spiral
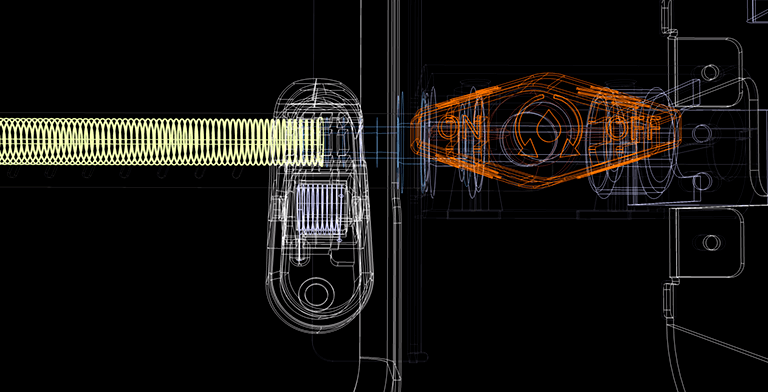
The gadget was created using the principles of applying polymer material and the element basis features of 3D printing equipment. However, owing to the unique characteristics of the application, it was required to construct a fundamentally new device, identify kinematic principles, and refine the component base for medical usage. Traditional printers use three-dimensional coordinates and cannot use spiral shapes. The technical team included an electronic stepper motor control module to enable rotation of the interior object (vascular bed) around its axis and synchronisation with the dosing and application of polymer material.
Polytetrafluoroethylene is the polymer (Teflon). Because of its biological compatibility with the human body, it is effectively employed in the fabrication of implants for cardiovascular and general surgery, dentistry, and ophthalmology. Teflon is regarded as the best material for the manufacture of artificial blood arteries and cardiac stimulants.
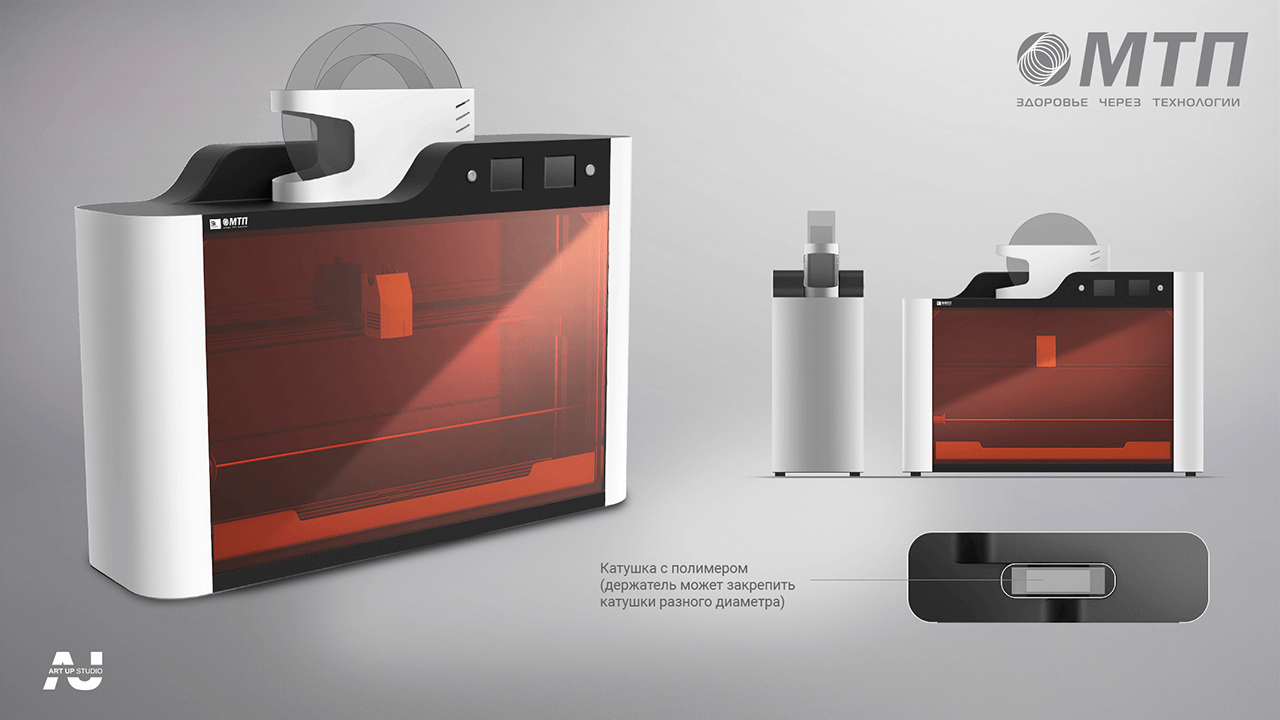
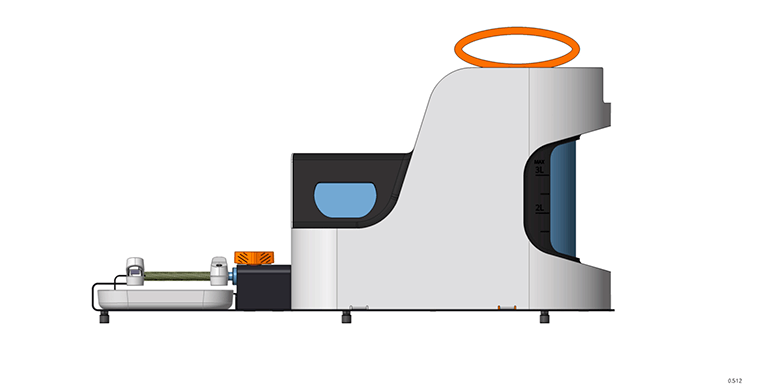
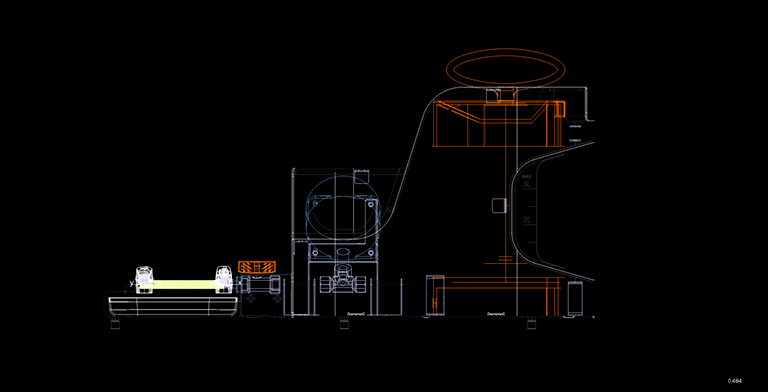
The overall size and internal arrangement of the device's functioning section established the important technical specifications, namely varied diameters ranging from 6 to 10 mm and the length of the vascular bed itself ranging from 80 to 400 mm.
Given these characteristics, it became important to assure the diversity of the applied spiral's size, which necessitated the creation of specific software with a control interface for defining the initial printing settings. To complete this work, a layout solution for storing specialist equipment—guide rods to support the vascular bed throughout the strengthening process—was necessary.
Industrial design
After determining the final layout of the future device, an analytical pre-project research was done to assess the scope of use and probable constraints of the hardware complex. The devices enable desktop operation in clean room laboratory settings, which served as the foundation for shape, styling, and colour solutions.
The designs for the future gadget were presented to the client, and the device's operating principle was offered. One of the critical steps was the selection of a stylistic solution, materials, and production technologies that would unite the line of future devices. The device's design is minimalistic. Functional aspects are accentuated in bright colours, making the device interface more intuitive and facilitating user engagement with them.
The gadget is placed in an aluminium container covered with powder paint that is suitable for contact in the fabrication of medical equipment, and it is also outfitted with plastic panels that are resistant to the impacts of hostile chemicals. As a result, all surfaces have greater resistance to disinfectants.
The work area is accentuated visually with contrasting orange and black colours. We devoted careful attention to ergonomics: a lifting mechanism was added to reach the work area, which efficiently and smoothly opens the device's door upwards. This design allows you to take up less space and makes working with the gadget more easy. A drawer under the working area stores the equipment for various diameters of prosthesis.
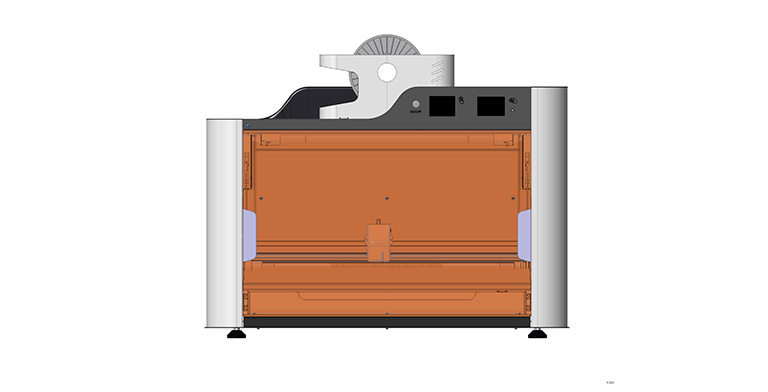
Construction site
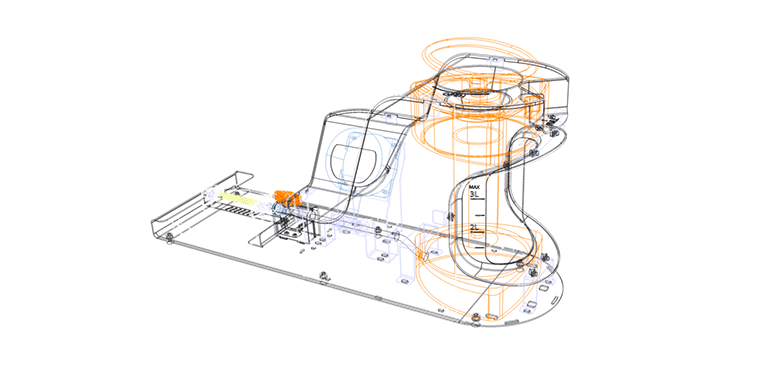
During the design phase, our experts devised a way for attaching a spiral to a tank using additive technologies and chose electronics to manage the device's operation. The device's casing was broken into components and worked out technologically. Standardized metal profiles were used to ensure the requisite printing accuracy and the stiffness of the device's frame, providing the device's low cost in small-scale manufacture (from 20 to 50 devices per year). The design was completed in CAD, which allows you to easily advance to the phases of technological development and product part production at any moment.
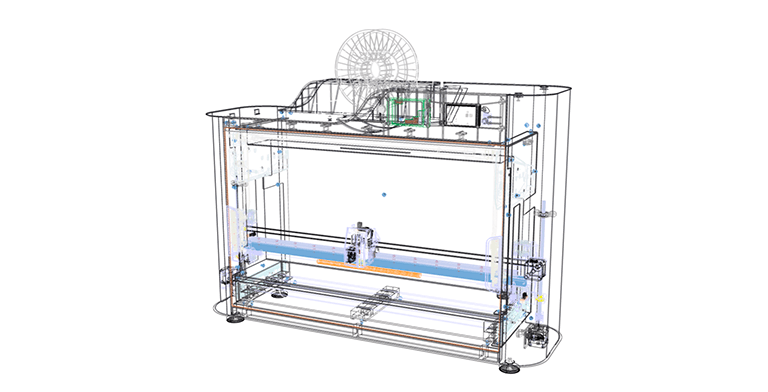
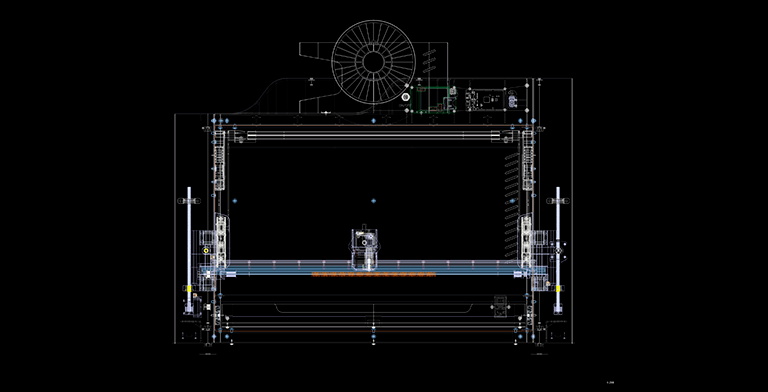
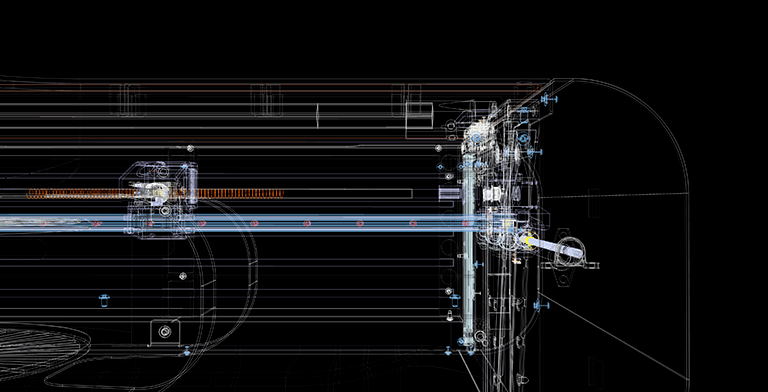
Software development
As part of the research, dedicated software was developed that controls the printing settings and lets the user to specify the starting point, spiral pitch, reinforcement length, rotation speed, and diameter of the prosthesis. Depending on the specifications of the strengthened vascular bed, these parameters alter and are changed on the touchscreen placed on the front of the device. As a result, the programme allows you to verify that the spiral enters the recess of the corrugation of vessels of varying diameters. In order to fix the spiral, the function of drawing the start and end turns of the spiral around the circle was introduced.
Equipment construction and assembly
For the fabrication of parts, ART UP Studio professionals chose the following technologies: laser cutting, bending, and powder coating for body metal parts. Three-dimensional milling was used to create the plastic components of the casing lining, which were then coated with a decorative and protective finish. Due to the low serial number, we were able to use 3D printing technologies: we printed out the details of fastening vessels for various diameters using our FormLabs Form2 SLA printer. This method has lowered the cost and production time.
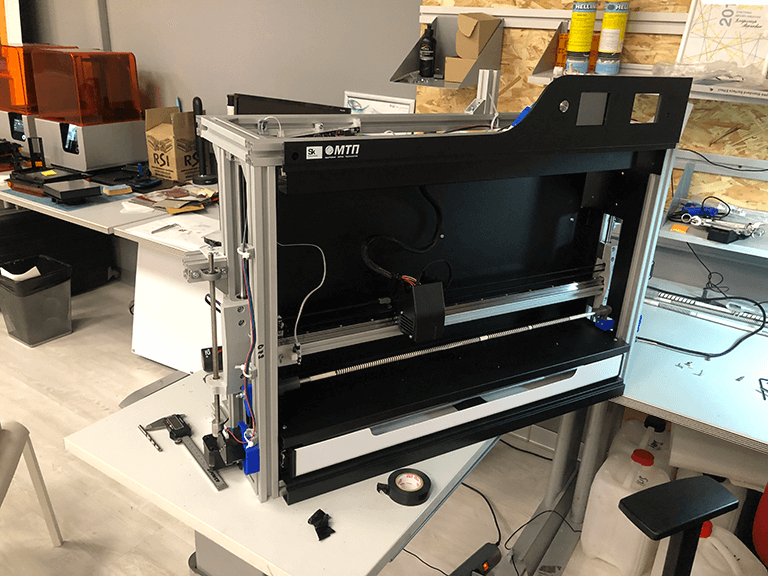
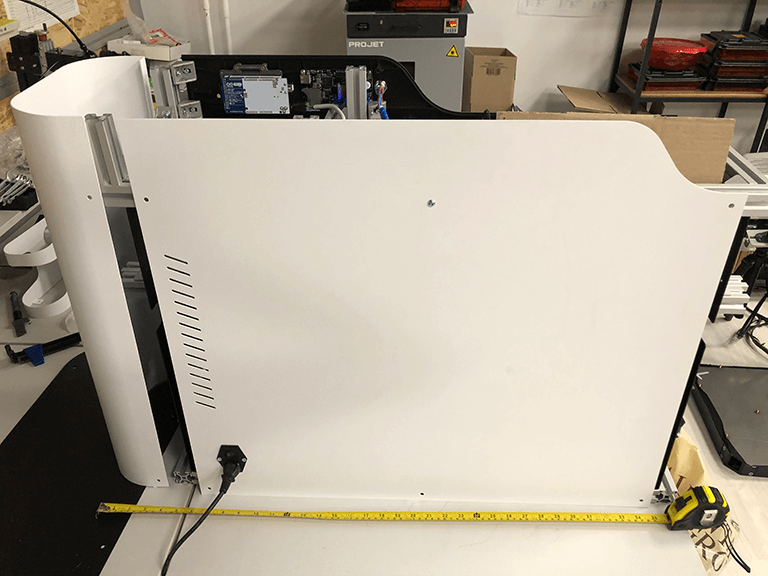
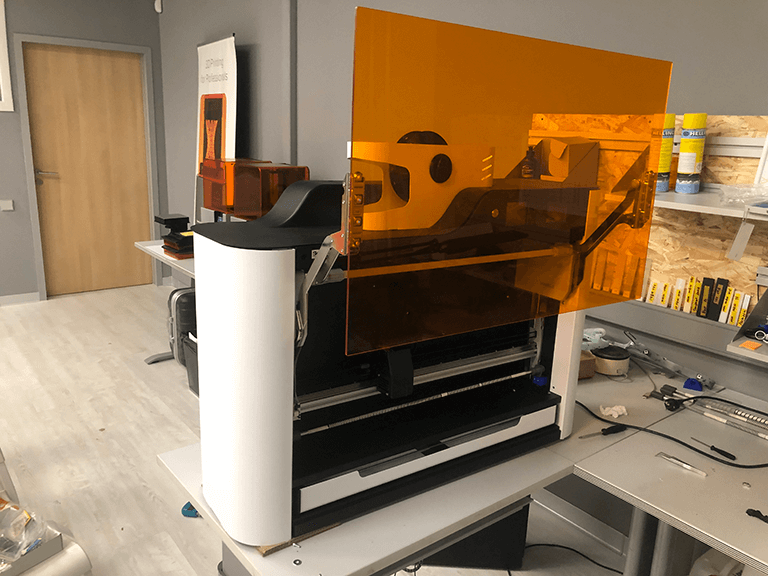

Device calibration and testing
At the end, our studio calibrated the gadgets and conducted many successful tests with the help of the customer's specialists. The key technological objective was completed: a biopolymer spiral produced by an extruder fits into the corrugation depression.The second product is a device for checking for porosity
Work that has been completed:
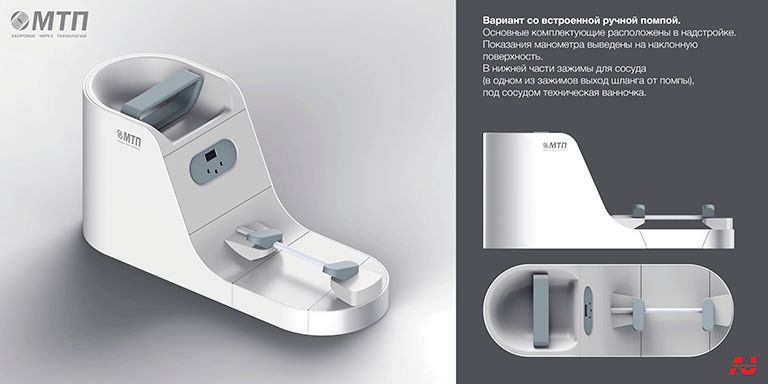
To test vascular prosthesis for tightness and leakage, it was required to incorporate a water pumping device, a pressure monitoring system, and clamps in a sealed system. Our studio's experts chose the most basic and understandable functioning steam operation - a mechanical water pump with a pressure gauge. These components have been obtained, which has greatly lowered the cost and quantity of parts for manufacture, decreased development time, and will further simplify equipment replacement and repair. Latches for securing the tested material and the water supply region, which included a sealed tap and a fixed metal holder, were designed as independent phases.
The major design feature - a mechanical pump - controlled the total proportions of the device, which necessitated the construction of a different scenario for the future gadget's use. The piston handle and a portion of the pump bulb were left exposed, which added practicality and ergonomics to the design while also improving the visual appearance.Industrial design
The design of the reinforcing printer was created in tandem with the first device in order to connect two disparate devices into a single complex. The device's look is equally simple, with smooth transitions and no apparent fasteners.
The buyer was given numerous alternatives regarding the concept of the future product. The aesthetic solutions, materials, and manufacturing technique chosen match to the first designed device for applying and fastening a polymer spiral. Functional aspects are also highlighted in bright orange, making it easier to use the gadget.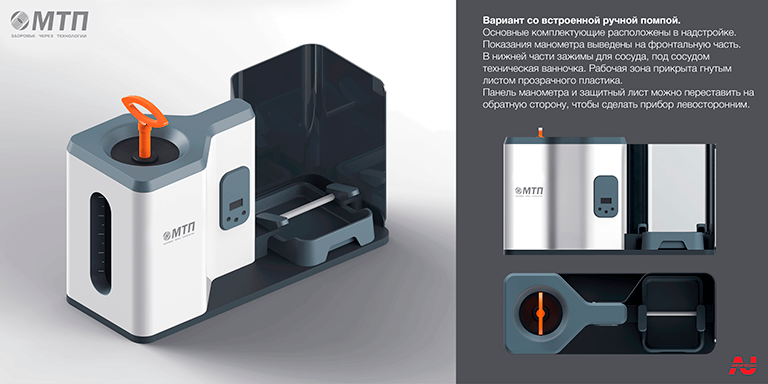
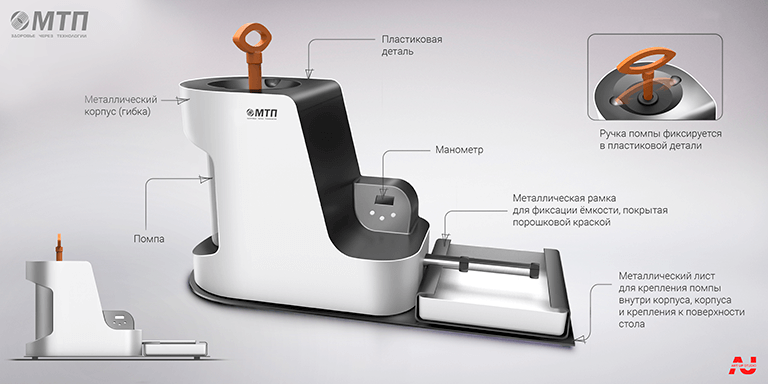
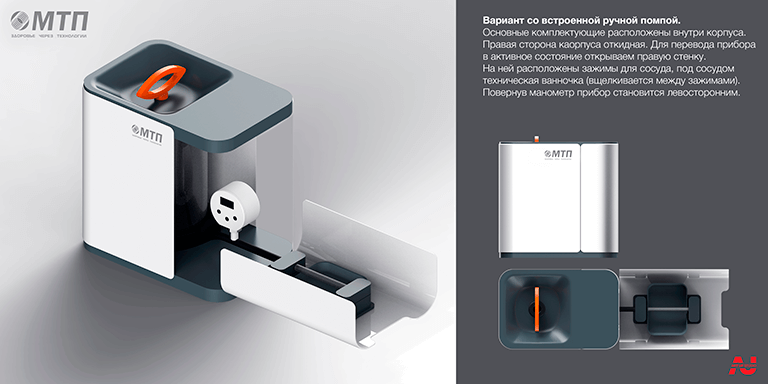
Conceptual search for an appearance device
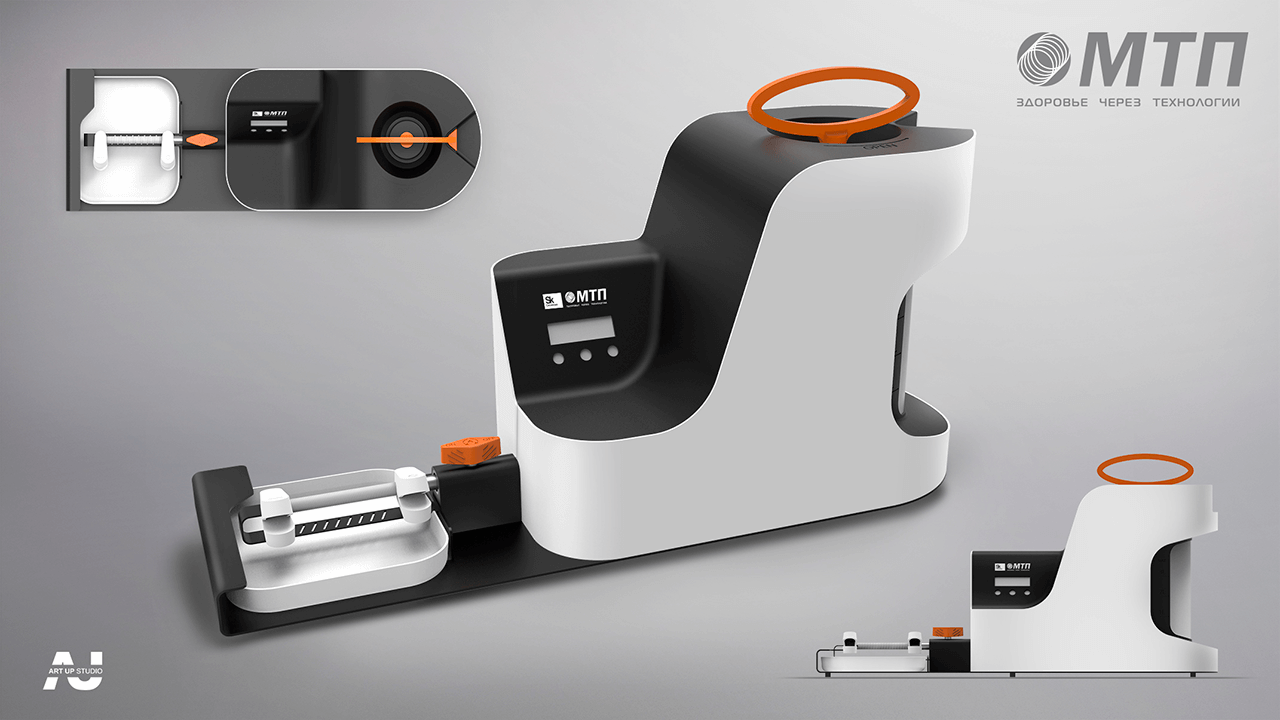
Final version
Construction site
The primary goal of the endeavour was to make the gadget intelligible, manageable, and dependable in operation. We separated the device into pieces and conducted a detailed design analysis on each portion, depending on the production processes chosen. The engineers at the studio chose standardised and acquired components, which allowed them to reduce the cost of producing specialty fasteners, electrical boards, frames, corners, touch screens, and power supply. At this point, the intricacies of the vascular prosthesis's attachment were created using a 3D printer (SLA) Formlabs Form2 constructed of a photopolymer mimicking rubber. The printed prototypes of the pieces allowed us to test and tweak the design aspects. Furthermore, the fixators were tested on the stand during the installation of prostheses of varied diameters.
Equipment manufacturing and assembly
The same manufacturing processes were used for the reinforcing printer as for the previous device: laser cutting, bending, and powder coating for case metal parts. As with the original device, the plastic elements of the casing lining were manufactured of ABS plastic by 3D milling and then coated with a decorative and protective coating.
The final features of the tooling and fixation of vascular prosthesis were made using 3D printing (SLA), which decreased the time for testing and confirming the assimilation of the device's vital components.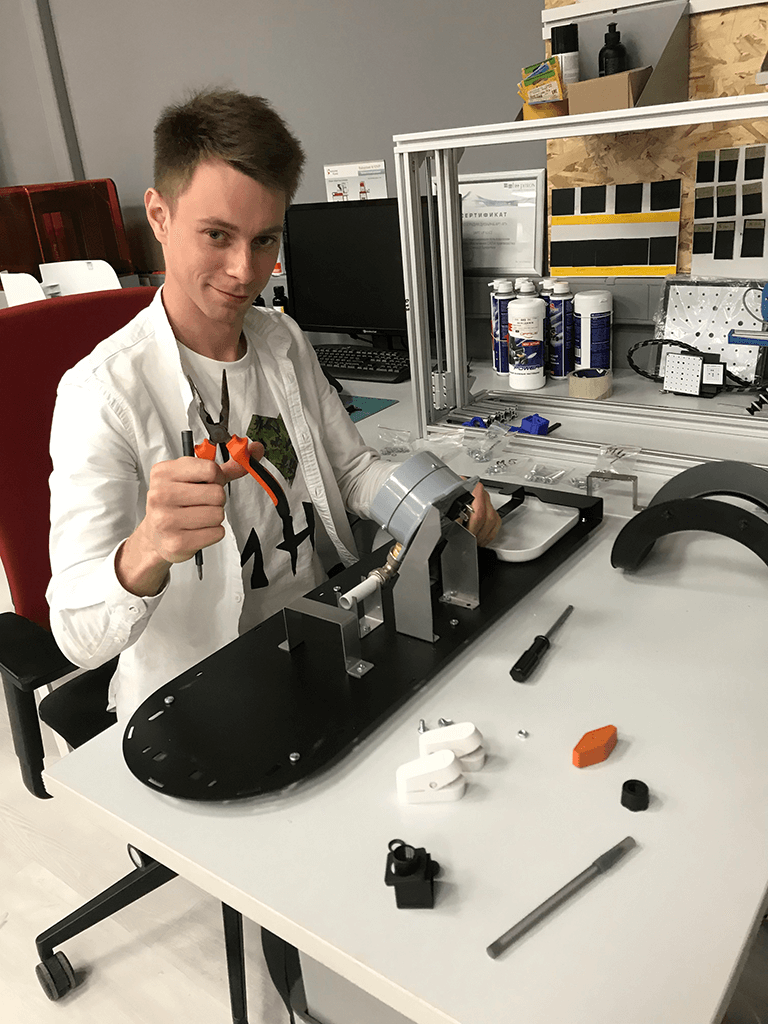


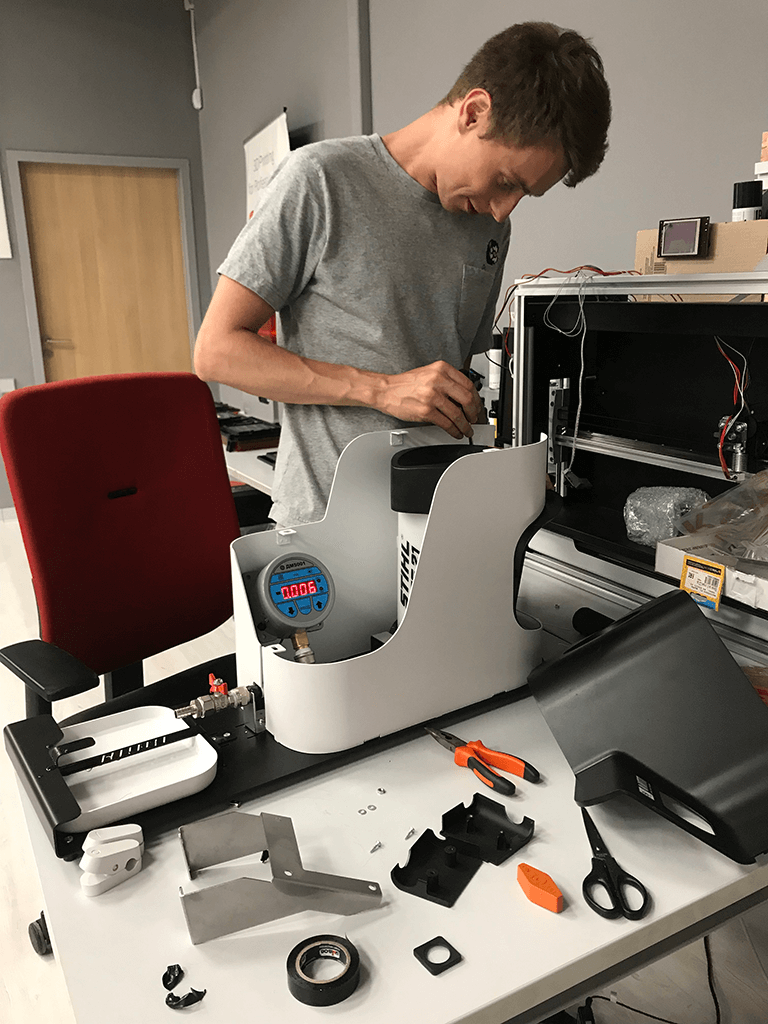


Device calibration and testing
Because the platform was meant to test reinforced containers for leakage, it was tested for proper operation inside the confines of our studio before final delivery. Each batch of ready-made vascular prosthesis had a little piece cut out of it, which was then treated with collagen and put on the device. The prosthesis's quality (non-admission of water) was evaluated at a pressure of 120 mmHg, which was pushed by a pump and measured with a pressure gauge.
A crucial measured parameter was the medical standard: the critical mark for the prosthesis is no more than 50 ml of water after 1 cm per minute.A high-quality product has very little porosity.
The end result of this laborious effort was a completely functional prototype that could be used to control the quality of vascular prosthesis.Results
The produced hardware complex was built in a single copy and fits the criteria set by the client, the requirements for medical equipment, and is also acceptable for work in the laboratory of the clean room of Medtechnoproekt LLC on the Skolkovo Technopark's property. In less than a year, our ART UP Studio team completed complicated engineering and design work, creating unique high-tech items that embody scientific achievements on the Skolkovo Innovation Center's campus.
For the first time, it was feasible to demonstrate the application of the popular trend "Additive Technologies" in the area of medicine for a rare problem that is in the early stages of development throughout the world-vascular reinforcement. This will enable the development of blood vessel prostheses to take a significant stride forward in the future.





Worked on the project:
Designer - Maria Nikitina
Design engineer - Vladimir Ternovykh
Software developer - Kirill Puchkov

 IF Design Award 2019-2021
IF Design Award 2019-2021
 Скачать бланк ТЗ
Скачать бланк ТЗ
 Презентация
Презентация